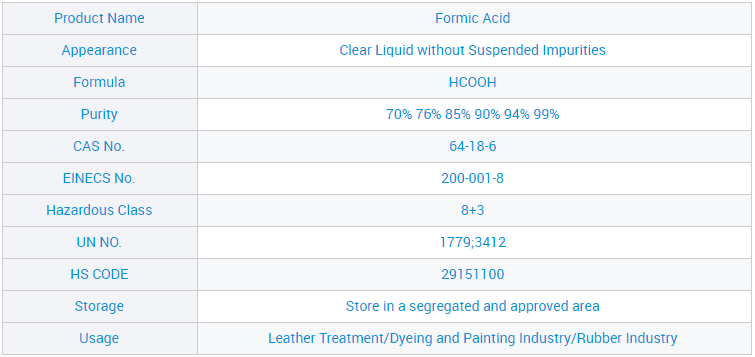
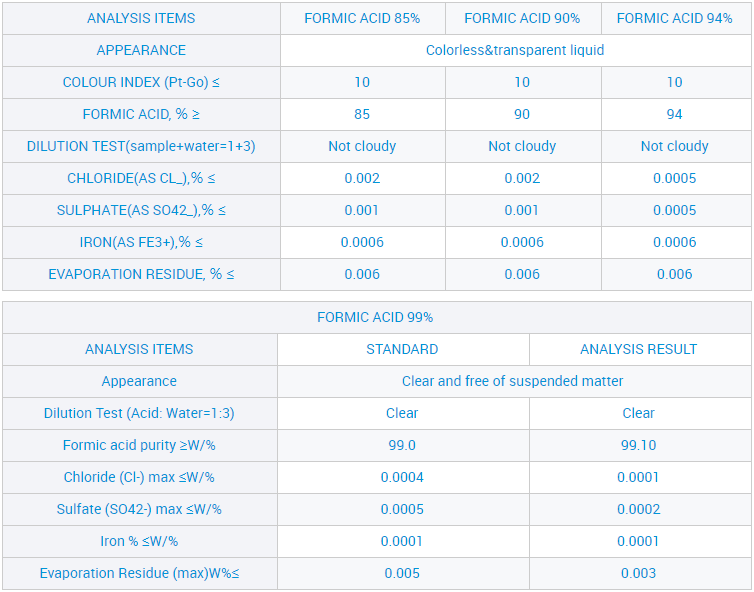
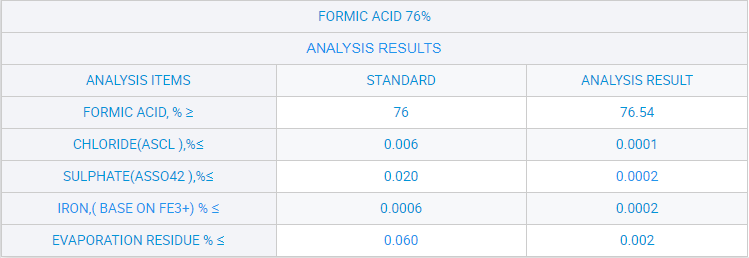
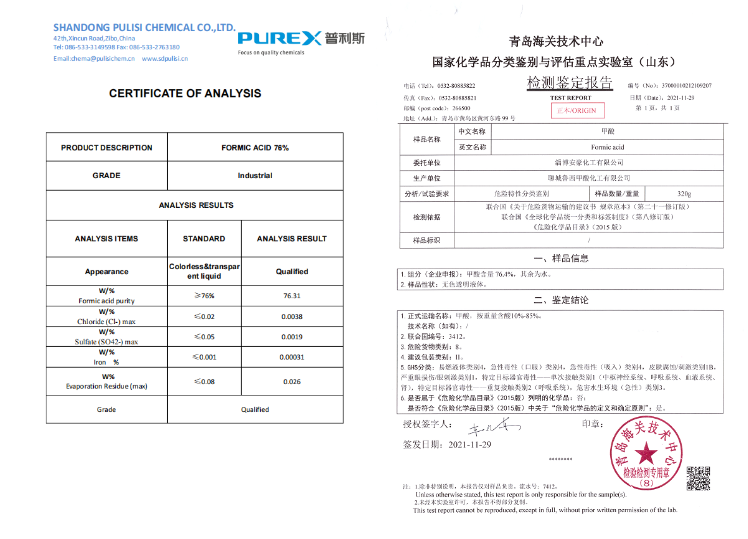
Liquid Formic Acid Leather Use
Our organization sticks for the principle of “Quality will be the life of your business, and name may be the soul of it” for Liquid Formic Acid Leather Use, We sincerely welcome buyers from both at your house and overseas to arrive to barter organization with us.
Our organization sticks for the principle of “Quality will be the life of your business, and name may be the soul of it” for , The working experience in the field has helped us forged a strong relations with customers and partners both in domestic and international market. For years, our products have been exported to more than 15 countries in the world and have been widely used by customers.












Frequently Asked Questions
Need help? Be sure to visit our support forums for answers to your questions!
Can we print our logo on the product ?
Of course, we can do it. Just send us your logo design.
Do you accept small orders?
Yes. If you are a small retailer or starting up business,We are definitely willing to grow up with you. And we are looking forward to co-work with you for a long term relationship.
How about the price? Can you make it cheaper?
We always take the customer’s benefit as the top priority. Price is negotiable under different conditions, we are assuring you to get the most competitive price.
Do you offer free samples?
It’s appreciated that you could write us positive reviews if you like our products and service, we will offer you some free samples on your next order.
Are you able to deliver on time?
Of course!we specialised in this line for many years,many customer make a deal with me because we can deliver the goods on time and keep the goods top quality!
Can I visit your company and factory in China?
Sure. You are very welcomed to visit our company in Zibo, China. (1.5H drive way from Jinan)
How Can I place an order?
You could just send us inquiry to any of our sales representatives to get detailed order information, and we will explain the detail process.Acide formique Reaction Mechanism
Acide formique Formic acid is produced through the oxidation of methanol. The chemical equation for this reaction is:
CH₃OH + 1.5 O₂ → HCOOH + H₂O
This reaction requires heating and oxygen as a reactant. In industrial production, air (containing ~21% O₂) is typically used as the oxygen source.



















